Carbon monoxide (CO) is produced by the combustion of fossil fuels and biomass, and by the oxidation of methane and other hydrocarbons. It is removed mainly through reaction with OH radicals in the atmosphere. CO is not a greenhouse gas in itself because it absorbs hardly any infrared radiation from the ground surface. However, as it is a precursor of tropospheric ozone, it can affect the concentrations of many greenhouse gases through reaction with OH radicals.
WDCGG analysis indicates that the globally averaged atmospheric CO mole fraction was 90 ppb in 2022.
JMA observes surface concentrations of CO at three stations in Japan. The figure below shows a time-series representation of CO mole fractions at the three stations. There are clear seasonal cycles with high mole fractions from winter to spring and low mole fractions in summer at all three stations. The increased figure for 1997 - 1998 suggests an influence from forest fires in Indonesia and Siberia. A smaller increase was also seen in 2002 - 2003.
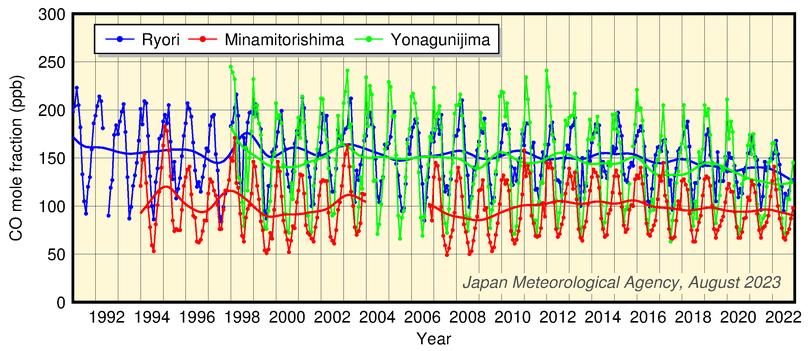
Time-series representation of CO mole fractions recorded at JMA observatories
Monthly mean mole franctions and those with seasonal variations removed are shown. Provisional values are included.
Data from Minamitorishima are missing due to equipment problems and typhoon damage from January 2004 to October 2006.
The figure below shows annual variations in the latitudinal distribution of CO mole fractions. There is a clear seasonal cycle with high mole fractions from winter to spring and low mole fractions in summer. The figures are high in mid and high latitudes in the Northern Hemisphere and low in the Southern Hemisphere, indicating that the major sources are distributed in the mid and high latitudes of the Northern Hemisphere. The fractions decrease toward tropical ocean areas, where CO is destroyed photochemically.
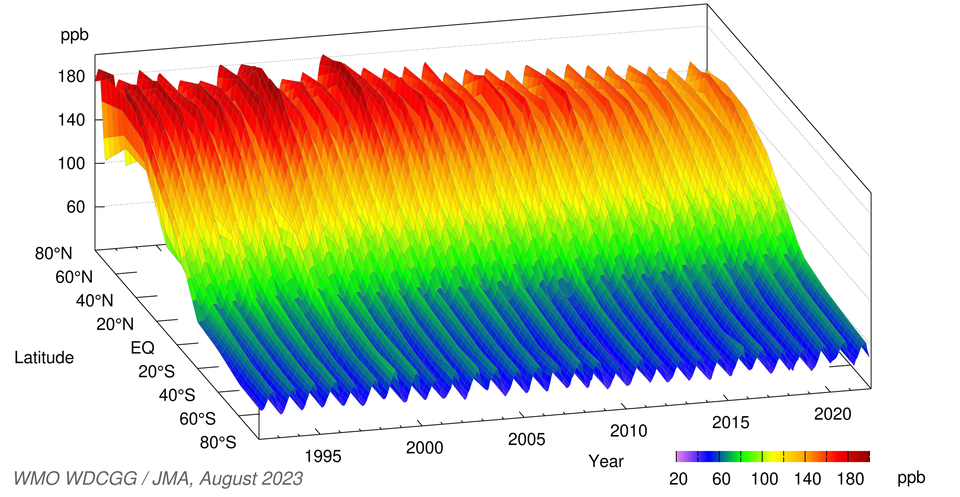
Time-series representation of zonally averaged CO mole fractions