Tropospheric ozone (O3) is produced by the photochemical oxidation of methane (CH4) , carbon monoxide (CO) and non-methane hydrocarbons (NMHCs) in the presence of rich nitrogen oxides (NOx) . It is removed through reaction with hydrogen oxides (HOx, i.e., HO2 and OH) . Tropospheric O3 is chemically reactive and can produce OH radicals, which affect atmospheric concentrations of CH4 and many other greenhouse gases through chemical reactions. Tropospheric O3 also acts as a significant greenhouse gas because it has strong absorption bands in the infrared wavelength range. In the mid and high latitudes, O3 is transported from the stratosphere down to the troposphere and destroyed on the ground surface.
JMA observes surface mole fractions of O3 at three stations in Japan.
The figure below shows a time-series representation of O3 mole fractions at the three stations. The data usually exhibit seasonal variations with maxima in winter to spring and minima in summer. The reduced presence of O3 in summer is attributed to increased OH radical concentrations resulting from increased water vapor presence stemming from high temperatures. While Yonagunijima and Minamitorishima are located in the same latitudinal zone, the mole fraction at the former is usually higher.
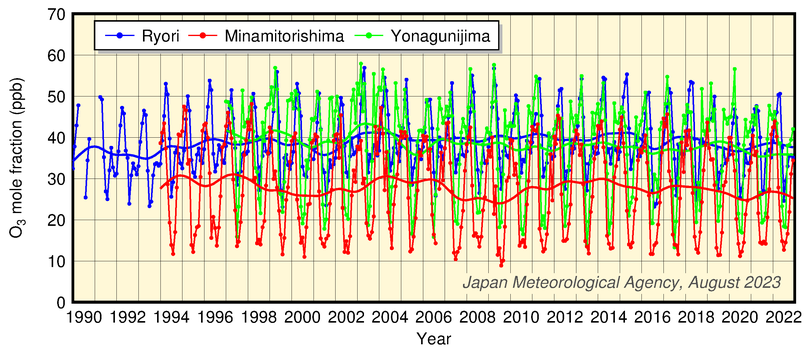
Time-series representation of surface O3 mole fractions recorded at JMA observatories
Monthly mean mole franctions and those with seasonal variations removed are shown.
Provisional values are included.